Vitamin K
Vitamin K denotes a group of 2-methilo-naphthoquinone derivatives. They are human vitamins, lipophilic (i.e., soluble in lipids) and therefore hydrophobic (i.e., poorly soluble in water). They are needed for the posttranslational modification of certain proteins, mostly required for blood coagulation.
Vitamin K2 (menaquinone, menatetrenone) is normally produced by bacteria in the intestines, and dietary deficiency is extremely rare unless the intestines are heavily damaged.
Chemical structure
Vitamin K is a group name for a number of related compounds, which have in common a methylated naphthoquinone ring structure, and which vary in the aliphatic side chain attached at the 3-position (see figure 1). Phylloquinone (also known as vitamin K1) invariably contains in its side chain four isoprenoid residues, one of which is unsaturated.
Menaquinones have side chains composed of a variable number of unsaturated isoprenoid residues; generally they are designated as MK-n, where n specifies the number of isoprenoids.
It is generally accepted that the naphthoquinone is the functional group, so that the mechanism of action is similar for all K-vitamins. Substantial differences may be expected, however, with respect to intestinal absorption, transport, tissue distribution, and bio-availability. These differences are caused by the different lipophilicity of the various side chains, and by the different food matrices in which they occur.
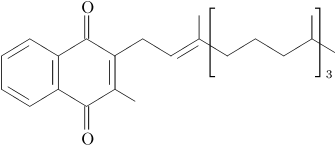 | 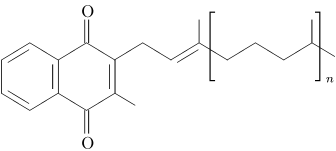 |
| Figure 1: Chemical structures of vitamin K1 (phylloquinone, left structure) and vitamin K2 (menaquinones, right structure). Both contain a functional naphthoquinone ring and an aliphatic side chain. Phylloquinone has a phytyl side chain, whereas in menaquinone the side chain is composed of a varying number of isoprenoid residues. | |
Physiology
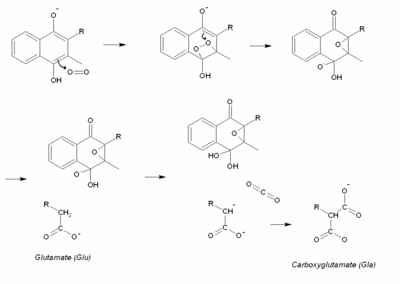
Vitamin K is involved in the carboxylation of certain glutamate residues in proteins to form gamma-carboxyglutamate residues (abbreviated Gla-residues). In the active site of vitamin K-dependent carboxylases, vitamin K is thought to react with oxygen yielding a very strong base This base can abstract a proton from the Glu sidechain, thus forming a carbanion that can easily react with CO2 and produce Gla.
Gla-residues are usually involved in binding calcium, and are essential for the biological activity of all known Gla-proteins.
At this time 14 human Gla-proteins have been discovered, and they play key roles in the regulation of three physiological processes:
- blood coagulation (prothrombin (factor II), factors VII, IX, X, protein C, protein S and protein Z)
- bone metabolism
- vascular biology.
In many bacteria vitamin K is involved in anaerobic respiration. Here it serves as an electron carrier from the reductant NADH to e.g. the oxidant TMAO
Recommended amounts
The U.S. Dietary Reference Intake (DRI) for an Adequate Intake (AI) for a 25-year old male for Vitamin K is 120 micrograms/day. No Tolerable Upper Intake Level (UL) has been set.
Role in disease
Vitamin K-deficiency may occur by disturbed intestinal uptake (such as would occur in a bile duct obstruction), by therapeutic or accidental intake of vitamin K-antagonists or, very rarely, by nutritional vitamin K-deficiency. As a result of the acquired vitamin K-deficiency, Gla-residues are not or incompletely formed and hence the Gla-proteins are inactive. Lack of control of the three processes mentioned above may lead to the following: risk of massive, uncontrolled internal bleeding, cartilage calcification and severe malformation of developing bone, or deposition of insoluble calcium salts in the arterial vessel walls.
History
Discovery
In the late 1920s, Danish scientist Henrik Dam investigated the role of cholesterol by feeding chickens a cholesterol-depleted diet. After several weeks, the animals developed hemorrhages and started bleeding. These defects could not be restored by adding purified cholesterol to the diet. It appeared that - together with the cholesterol - a second compound had been extracted from the food, and this compound was called the coagulation vitamin. The new vitamin received the letter K because the initial discoveries were reported in a German journal, in which it was designated as Koagulationsvitamin. Edward Adelbert Doisy (of Saint Louis University) did much of the research that led to the discovery of the structure and chemical nature of Vitamin K. Dam and Doisy shared the 1943 Nobel Prize for medicine for their work on Vitamin K. Louis Fieser was the first to synthesize the compound.
For several decades the vitamin K-deficient chick model was the only method of quantitating of vitamin K in various foods: the chicks were made vitamin K-deficient and subsequently fed with known amounts of vitamin K-containing food. The extent to which blood coagulation was restored by the diet was taken as a measure for its vitamin K content.
The first published report of successful treatment with vitamin K of life-threatening hemorrhage in a jaundiced patient with prothrombin deficiency was made in 1938 at the University of Iowa Department of Pathology by Drs. Harry Pratt Smith, Emory Warner, Kenneth Brinkhous, and Walter Seegers.
The precise function of vitamin K was not discovered until 1974, when Stenflo et al isolated the vitamin K-dependent coagulation factor prothrombin (Factor II) from cows that had received a high dose of the vitamin K antagonist warfarin. It was shown that normal prothrombin contained 10 unusual amino acid residues which were identified as gamma-carboxyglutamate. Prothrombin isolated from warfarin-treated cows had normal glutamate at the Gla-positions and was designated as descarboxyprothrombin. The extra carboxyl group in Gla made clear that vitamin K plays a role in a carboxylation reaction during which Glu is converted into Gla.
Gla-proteins
At present, the following human Gla-proteins have been characterized to the level of primary structure: the blood coagulation factors II (prothrombin), VII, IX, and X, the anticoagulant proteins C and S, and the thrombin-targeting protein Z, the bone Gla-protein osteocalcin, the calcification inhibiting matrix gla protein (MGP), the cell growth regulating growth arrest specific gene 6 protein (Gas6), and the four transmembrane Gla proteins (TMGPs) the function of which is at present unknown. Gas6 can function as a growth factor that activates the Axl receptor tyrosine kinase and stimulates cell proliferation or prevents apoptosis in some cells. In all cases in which their function was known, the presence of the Gla-residues in these proteins turned out to be essential for functional activity.
Gla-proteins are known to occur in a wide variety of vertebrates: mammals, birds, reptiles, and fish. The venom of a number of Australian snakes acts by activating the human blood clotting system. Remarkably, in some cases activation was accomplished by Gla-proteins capable of binding to phospholipid membranes and subsequent conversion of procoagulant clotting factors into activated ones.
Another interesting class of invertebrate Gla-proteins is formed by the conotoxins, produced by the fish-hunting snail Conus geographus. These snails produce a neurotoxin containing a variety of extremely Gla-rich peptides, which are sufficiently powerful to kill an adult human.
Use On New-Born Babies
In some countries, injections of Vitamin K are routinely given to new born babies. Vitamin K is used as prophylactic measure to prevent late-onset haemorrhagic disease (HDN). However, HDN is a relatively rare problem, and many parents now choose for their babies not to have such an injection.
References
- Dam H. The antihemorrhagic vitamin of the chick. Occurrence and chemical nature. Nature 1935;135:652.
- Stenflo J, Fernlund P, Egan W, Roepstorff P. Vitamin K dependent modifications of glutamic acid residues in prothrombin. Proc Natl Acad Sci USA 1974;71:2730–3. PMID 4528109.